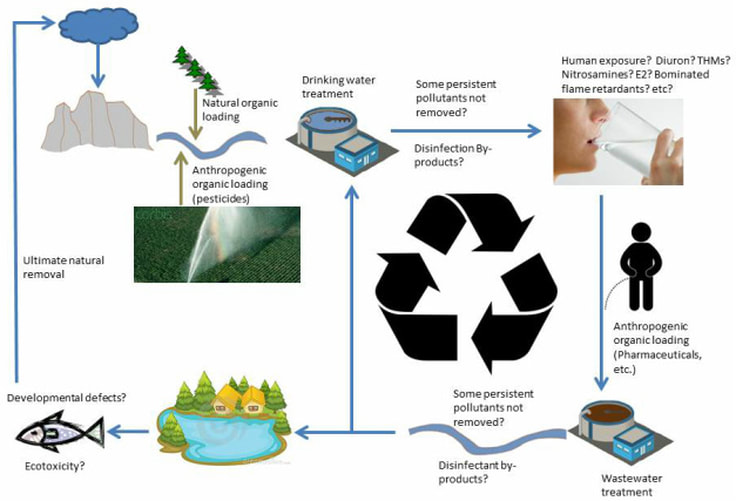
The Engineered Water Cycle
Increasing global population has amplified anthropogenic loading of organic matter to the water cycle, and increased the rate of incidental water reuse, both of which are core concepts of my team's research. We focus on environmental organic and inorganic chemistry and process based water treatment. Descriptions of several funded projects are below, but, the current focus of the lab is on:
1) Direct/indirect Potable Reuse. As our supplies of pristine drinking water sources dwindle, we will have to rely on less conventional sources of water, such as wastewater. If the International Space Station does it, why can't we? The challenges we specifically wish to address include removal of organic and inorganic constituents such as pharmaceuticals, heavy metals, and pathogens.
2) Wildfire impacts to drinking water quality. The goal of this research is to understand how wildfire impacts downstream drinking water intakes and the effects to drinking water quality. Current work is focusing on recent Sierra fires near Auburn, CA, and South Lake Tahoe, CA.
3) Water as a Medium to Partition and Remove CO2 from the Atmosphere. The greenhouse gas CO2 naturally partitions to the aqueous phase. We aim to use novel materials to sequester CO2. This will reduce the concentration of CO2 in the water and will drive further partitioning, removing CO2 from the atmosphere.
4) Anthropogenic Chemicals in the Environment. My broad interest is in the sources, fate, and impacts of anthropogenic chemicals in/on the environment. Research focuses on developing new analytical tools to quantify humankind's influence, and engineering tools to mitigate impact.
1) Direct/indirect Potable Reuse. As our supplies of pristine drinking water sources dwindle, we will have to rely on less conventional sources of water, such as wastewater. If the International Space Station does it, why can't we? The challenges we specifically wish to address include removal of organic and inorganic constituents such as pharmaceuticals, heavy metals, and pathogens.
2) Wildfire impacts to drinking water quality. The goal of this research is to understand how wildfire impacts downstream drinking water intakes and the effects to drinking water quality. Current work is focusing on recent Sierra fires near Auburn, CA, and South Lake Tahoe, CA.
3) Water as a Medium to Partition and Remove CO2 from the Atmosphere. The greenhouse gas CO2 naturally partitions to the aqueous phase. We aim to use novel materials to sequester CO2. This will reduce the concentration of CO2 in the water and will drive further partitioning, removing CO2 from the atmosphere.
4) Anthropogenic Chemicals in the Environment. My broad interest is in the sources, fate, and impacts of anthropogenic chemicals in/on the environment. Research focuses on developing new analytical tools to quantify humankind's influence, and engineering tools to mitigate impact.
Active Research
Understanding Wildfire Risks to Drinking Water Source Waters: Pyrogenic Changes to Organic Matter and Disinfection By-product Formation (Funded by NSF)

Wildfires are growing in size, severity, and frequency due to drought, forest management, and climate change. Wildfire mobilizes organic matter through partial combustion and pyrolysis. This pyrogenic organic matter is substantially different in composition from naturally occurring organic matter and it is transported through overland flow to downstream drinking water treatment. Disinfection is a key component of drinking water treatment and inactivates microorganisms by oxidation. However, the oxidants used also react with the diverse organic structures present in natural organic matter and some of these reactions result in the production of carcinogenic disinfection by-products, which have regulatory limits in finished drinking water set by the U.S. Environmental Protection Agency. Published literature has reported both, that pyrogenic organic matter is less reactive than naturally occurring organic matter in forming disinfection by-products, and also that pyrogenic organic matter is more reactive in forming disinfection by-products. The specific changes to the organic matter and what causes this divergence after fire are not clear. The goal of this research is to understand how fire severity affects DBP formation.
Thermal Regeneration of PFAS-laden Granular Activated Carbon presents an Opportunity to Break the Forever PFAS Cycle (Funded by NSF in collaboration with the University of Maine)
Pollution of the natural environment with per- and poly-fluorinated alkyl substances (commonly known as PFAS) is an overwhelming ecological and human health crisis. PFAS are toxic at low exposure levels and are difficult to destroy. Because they are difficult to destroy, solutions to PFAS contamination focus on removal from water, soil, and air, rather than destruction. One technique to remove PFAS from both air and water is adsorption to activated carbon. Activated carbon has already been demonstrated at scale for removal of PFAS. Consequently, thousands of activated carbon filters have been installed across the nation. This trend is likely to continue. However, activated carbon filters retain, but do not destroy PFAS which are removed from water or air. The spent filters can then act as PFAS sources if not disposed of properly. The goal of this research is to examine if thermal regeneration of activated carbon is a viable strategy to destroy retained PFAS while renewing the exhausted filter. There is little knowledge of PFAS destruction mechanisms and products during carbon regeneration. A lack of understanding of PFAS transformation and destruction during thermal regeneration may lead to rerelease of captured PFAS. The knowledge gained in this project will serve the public because it will facilitate the end to PFAS circulation in the environment. PFAS are estimated to impact at least 200 million U.S. citizens. A complete approach to destroy PFAS while also regenerating GAC will result in broad societal impacts with minimal or no new infrastructure investment.
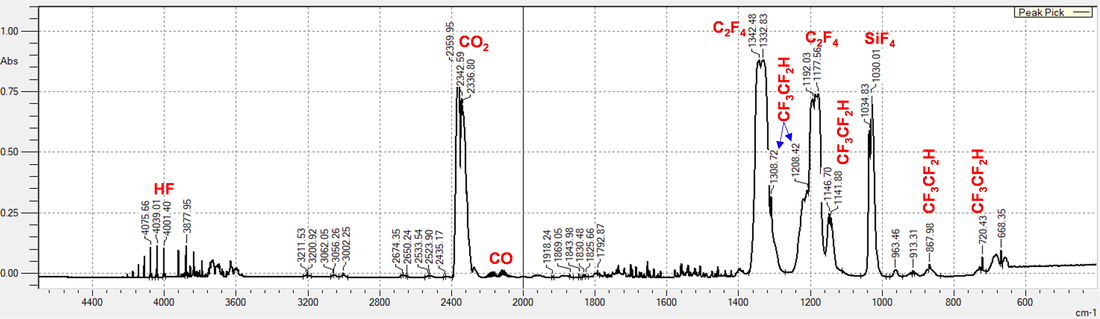
Rapid Site Profiling of Organofluorine: Quantification of PFASs by Combustion Gas Analysis (Funded by SERDP)
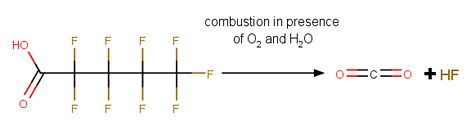
Per- and polyfluoroalkyl substances (PFASs) are released to the environment through several pathways, including use as aqueous film-forming foams for fire-control by the U.S. military. The U.S. Environmental Protection Agency has set health advisory levels for two PFASs based upon environmental persistence and adverse health outcomes and multiple U.S. military bases and airports are subsurface contaminated with PFASs. Sampling and quantifying PFASs is required to remediate contaminated sites, but the process is time-consuming, requires costly instrumentation and expertise (LC-MS/MS), and fails to capture many organofluorine transformation products and precursors with likely health impacts to exposed aquatic species and humans. Our goal is to develop and validate robust, field-ready instrumentation and methods to quantify total organofluorine. The method is based on combustion of PFASs to HF, and measurement of HF.
Perfluorinated Substance Release to Air (funded by NSF)
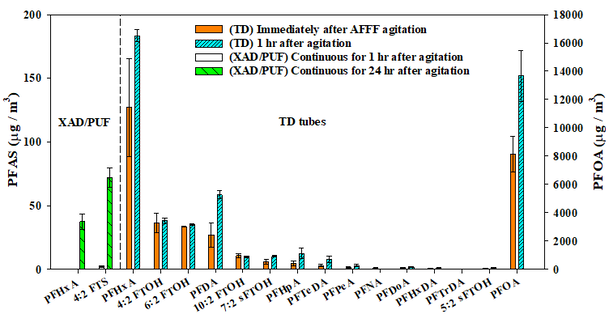
Research on per- and polyfluoroalkyl substances (PFASs) released from aqueous film-forming foams (AFFFs) has primarily focused on soil and groundwater contamination, or atmospheric transport. However, gas phase PFAS release from AFFF has not been well examined. We investigated the presence of volatile PFASs in the headspace above agitated AFFF concentrate produced within the past two years using two analytical techniques. One method utilized polyurethane foam and XAD resin with liquid chromatography mass spectrometry to quantify 30 PFASs and is similar to methods used by others to measure PFASs in air. A second, more exploratory approach used a thermal desorption sampler and gas chromatography and mass spectrometry (GC-MS) to measure 22 PFASs. Sixteen PFASs were detected in the headspace, including five fluorotelomer alcohols (0.5−38.1 μg/m3), 10 perfluorinated carboxylic acids (0.4−13670 μg/m3), and one fluorotelomer sulfonate (72.1 μg/m3). The most abundant PFAS detected in the headspace was perfluorooctanoic acid (13670 μg/m3), although it was detected only by GC-MS. Five additional fully fluorinated, iodinated, and ethenyl fluorocarbons were identified but not quantified. It is likely that firefighters are exposed to these compounds, but the risk is not yet known.
Prior Research (but I am still interested in!)
Identification and Removal of N-nitrosodimethylamine Precursors from Drinking and Wastewater (Funded by NSF. Prior funding from WRF)
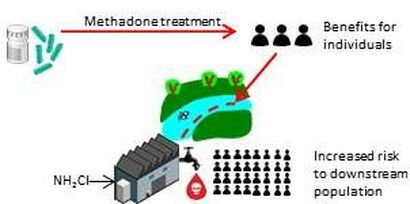
We attempted to remove NDMA precursors from wastewater using powdered and granular activated carbon (PAC and GAC) without knowing their exact chemical structure, using NDMA formation potential (FP) as a surrogate for precursor removal. In addition, we attempted to sorb some NDMA yielding pharmaceuticals to PAC. We found that NDMA FP removal was always better than dissolved organic carbon and UV254, indicating potential for NDMA precursors to act as "trace" organic compounds. Other research by us has shown that one of these trace organic compounds is methadone which is excrete in the urine and feces of individuals, but is later a potent NDMA precursor in wastewater and surface waters.
Impacts from Emerging Contaminants in Reclaimed Water used for Urban and Per-Urban Agriculture (Funded by USDA)
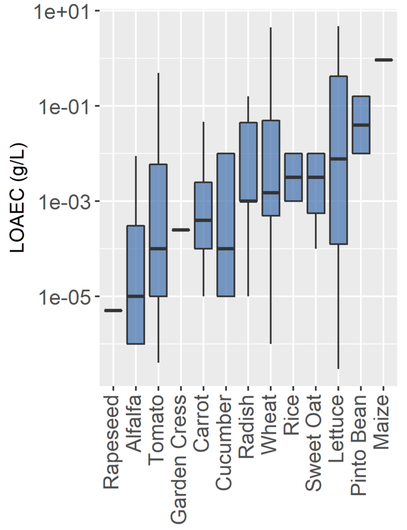
Wastewater from domestic and industrial sources is a resource for urban and peri-urban irrigated agriculture because it can provide both water and nutrients which are critical inputs to food production. But wastewater contains anthropogenic chemicals including pharmaceuticals and endocrine disrupters, which may pose a risk to grazing animals or humans. Research has been conducted in greenhouses and at bench-scale to assess these risks, but little research has been conducted at the field-scale with reclaimed wastewater used as the irrigation water. Our goal was to identify chemical contaminants in reclaimed water used for urban and peri-urban irrigated agriculture (forage crop and animal production); determine pathways (namely, water, soils, and sediments) of contaminant entrainment into agricultural products; determine associated health risks; and develop strategies for mitigation of those risks over the agricultural production chain, particularly focusing on reclaimed water production and point-of-use
Figure - Boxplot of lowest observable adverse effect concentrations for crops exposed to various individual xenobiotics from 25 publications using either hydroponics or watered soil as the exposure medium. Only publications with growth endpoints studying food crops or selected forage crops (e.g., alfalfa) exposed to municipal wastewater relevant xenobiotics are included. Individual xenobiotics are typically present in treated wastewater at <1e^-5 g/L in treated water.
Figure - Boxplot of lowest observable adverse effect concentrations for crops exposed to various individual xenobiotics from 25 publications using either hydroponics or watered soil as the exposure medium. Only publications with growth endpoints studying food crops or selected forage crops (e.g., alfalfa) exposed to municipal wastewater relevant xenobiotics are included. Individual xenobiotics are typically present in treated wastewater at <1e^-5 g/L in treated water.
Comparison of Environmental Stress Induced by Organic Chemical and Physical Sunscreens (Funded by EPA)
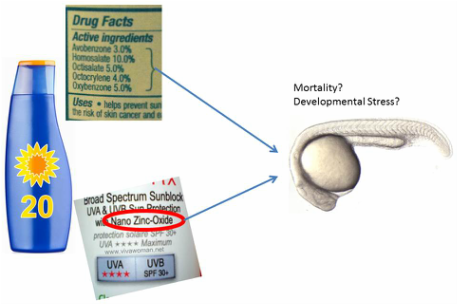
Sunscreens contain either an organic chemical UV filter (oxybenzone and others) or a physical sun barrier, typically nano-scale TiO2 or ZnO because these nanomaterials are nearly transparent to visible light when applied properly. Several organic chemical filters have been shown to cause photo-sensitization of the skin when exposed to light (likely through a reactive oxygen species [ROS] pathway) and are known to cross the epidermis into the blood, where they are eventually hepatically cleared. On the other hand, both TiO2 and ZnO are photo-active, which produce DNA damaging ROS when exposed to UV light such as that from the sun. Proprietary formulations of nano-containing sunscreens aim to either cease reactive oxygen species from being formed by coating the TiO2 or ZnO in aluminum or silica polymer, or quench the ROS as it is produced, presumably before it damages DNA. However, environmental release and transformation of these particles can cause them to be separated from their aluminium shell or ROS quencher, and produce environmental ROS that likely causes stress to aquatic ecosystems.
To-date, research assessing the impact of physical barrier (nano) sunscreens on ecosystems has been conducted using nanomaterials purchased from manufacturers with no guarantee of environmental relevance (use in actual sunscreens). Therefore, I have developed a method to extract nanomaterials from sunscreens. As of December, 2015, I am working to characterize these particles at Arizona State University using TEM with energy disspersive X-ray (EDX), single particle inductively coupled plasma mass spectrometry (spICP-MS), and laser induced breakdown spectroscopy (LIBS). In the near future I will work with collaborators at Oregon State University to compare the zebrafish toxicity and developmental stress caused by 10 nano sunscreen extracts to 5 FDA approved and 5 EU approved active organic sunscreen chemicals.
To-date, research assessing the impact of physical barrier (nano) sunscreens on ecosystems has been conducted using nanomaterials purchased from manufacturers with no guarantee of environmental relevance (use in actual sunscreens). Therefore, I have developed a method to extract nanomaterials from sunscreens. As of December, 2015, I am working to characterize these particles at Arizona State University using TEM with energy disspersive X-ray (EDX), single particle inductively coupled plasma mass spectrometry (spICP-MS), and laser induced breakdown spectroscopy (LIBS). In the near future I will work with collaborators at Oregon State University to compare the zebrafish toxicity and developmental stress caused by 10 nano sunscreen extracts to 5 FDA approved and 5 EU approved active organic sunscreen chemicals.